Diagnosis of myelodysplastic syndromes (MDS) and accurate risk assessment to tailor treatment remains challenging. High-risk MDS (hrMDS) patients who are fit enough may benefit from intensive chemotherapy followed by post-remission consolidation. The HOVON-SAKK (HO) acute myeloid leukemia (AML) trials, designed to test novel drug combinations with an intensive treatment regimen, included hrMDS patients (RAEB and RAEB-T). Since these HO-trials have been executed with large patient cohorts for many years, they offer the unique possibility to study the outcome, risk classification, and relevance of measurable residual disease (MRD) in hrMDS. A pooled database is available from HOVON-SAKK of patients who were treated with intensive chemotherapy (studies containing MDS patients with IPSS ≥ 1.5 HO42A, HO81, HO92, HO102, and IPSS-R > 4.5 HO103, HO132).
The database contained 3268 patients; 11% (364/3268) were hrMDS scored according to IPSS and IPPS-R. Three-year overall survival (OS) for hrMDS was 32% (standard error; SE 2%) compared to AML 46% (SE 1%). In total, 1074 AML and 75 hrMDS had reached complete remission (CR/CRi) and received a second induction cycle (C2). MRD after C2 based on multiparameter flow cytometry (Cloos et al., 2018, J. Vis.) with a cut-off of ≥0.1% for MRD positivity was assessed to evaluate the MRD status. We observed a trend of a higher MRD positivity rate for MDS compared to AML patients (MDS vs. AML: 32% (23/73) vs. 22% (232/1064), P = 0.055). Kaplan-Meier OS analysis showed a significant prognostic value of MRD in MDS, but not in the cumulative incidence of relapse (CIR) (log-rank test 0.011, Gray's test P = 0.124).
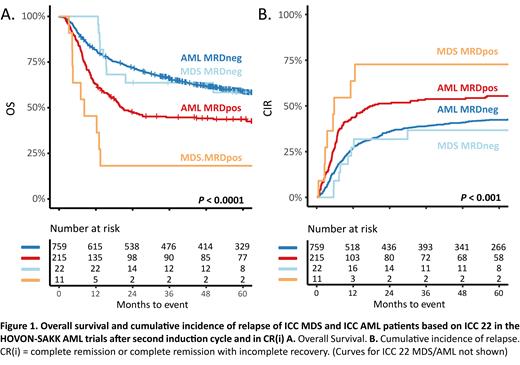
Since the MDS classification has been changed, we re-classified MDS based on the ICC 2022 classification into AML, AML/MDS, and MDS. We reclassified 3035 patients at diagnosis, including 2605 AML, 258 MDS/AML, and 172 MDS. The three-year OS was 47% (SE 1%) for AML, 29% (SE 3%) for MDS/AML, and 33% (SE 4%) for MDS. After C2, 2215 patients had reached CR/CRi. A total of 1074 patients containing 984 AML, 57 AML/MDS, and 33 high-risk MDS patients with CR/CRi after C2, with a suitable bone marrow sample, were included in the MRD analysis. Most patients were treated according to HO132 (40-48%). As expected, MDS patients were older (median age, range; 60, 32-74), had lower bone marrow blast counts, and were predominantly of adverse risk. A trend of a higher proportion of MRD positivity after C2 was observed in the MDS group (33%; 11/33) as compared to AML (22%; 215/984) or MDS/AML (18%; 10/57). Three-year OS was 61% for AML (SE 1.6%), 42% (SE 6.6%), and 49% (8.7%) for AML/MDS and MDS, respectively (Figure 1A). Kaplan-Meier OS analysis and CIR showed a significant prognostic value of MRD for MDS (log-rank test P<0.001, Gray's test P = 0.015) and AML ( P<0.001; P<0.001) but not for MDS/AML patients ( P = 0.5, P = 0.342)(Figure 1B). Remarkably, the three-year OS of MRDpos in MDS was 18% (SE 12%) compared to MRDpos in AML 40% (SE 16%).
The limitation of this study is the relatively small number of MDS patients according to the new ICC 2022 guidelines. In summary, MRD detection in the HOVON-SAKK trials shows the potential of MRD as a prognostic value in MDS patients treated on intensive induction regimens with a clinically validated and standardized assay.
Disclosures
Gjertsen:BerGenBio: Consultancy; GreinDX: Consultancy; Immedica: Consultancy; InCyte: Consultancy; Mendus AB: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Otsuka: Consultancy; Pfizer: Consultancy, Research Funding; Sanofi: Consultancy; in Alden Cancer Therapy AS: Current holder of stock options in a privately-held company; KinN Therapeutics AS: Current holder of stock options in a privately-held company; Coegin: Consultancy. Griškevičius:Miltenyi Biomedicine: Membership on an entity's Board of Directors or advisory committees. Juliusson:Servier: Honoraria; Novartis: Honoraria; Laboratoire Delbert: Other: Research cooperation; Jazz: Honoraria; AbbVie: Honoraria. Alhan:BMS: Membership on an entity's Board of Directors or advisory committees. de Leeuw:Roche: Consultancy; Takeda: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ellipses Pharma: Research Funding. Ossenkoppele:Abbvie: Consultancy; Servier: Consultancy; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Research Funding; BMS/Celgene: Consultancy, Honoraria; Janssen: Consultancy; AGIOS: Consultancy, Honoraria; Amgen: Consultancy; Gilead: Consultancy; Astellas: Consultancy, Honoraria; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; JazzPharmaceuticals: Consultancy. Cloos:BD Biosciences: Patents & Royalties: Royalties LSC tube; Navigate: Consultancy, Patents & Royalties: Royalties MRD assay; Novartis: Consultancy, Research Funding; Takeda: Research Funding. van de Loosdrecht:Roche: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding.